Clinical Trials
As the only academic healthcare system in San Diego County and one of few hospitals in the nation with the capabilities required to advance surgical innovation, the Department of Neurological Surgery is committed to advancing innovative treatments and procedures to ensure patients receive the best care possible.
We are proud that many studies conducted at UC San Diego have set national quality standards, advanced path-defining approaches and led to technologies now used routinely by neurosurgeons, including mechanical thrombectomy for stroke and endoscopic evacuation of intracranial hemorrhage (ICH). UC San Diego is evaluating a first-in-human neural stem cell transplantation for chronic spinal cord injury and advancing groundbreaking therapies and techniques, including immunotherapies, oncolytic viruses, laser interstitial thermal therapy (LITT) and more.
We look forward to continuing to advance innovations that will save lives, reverse disability and improve processes of care.
Brain Tumors
Gleolan for Visualization of Newly Diagnosed or Recurrent Meningioma (MEN-301)
Principal Investigator: Marc Schwartz, MD
Objective: Investigate the safety, diagnostic performance, and clinical usefulness of an imaging agent for the real-time detection and visualization of meningiomas during tumor resection surgery.
Eligibility Criteria: Adults with suspected meningioma or suspected recurrence of a meningioma
ClinicalTrials.gov Identifier: NCT04305470
Study Status: Enrolling
Contact: Jeff Mills, jhmills@health.ucsd.edu
Spine
Evaluating and establishing the relationship in the five critical X-ray time points in spinal deformity realignment: preoperative standing and supine, intraoperative pre- and post-correction, and postoperative standing films
Principal Investigator: Joseph Osorio, MD, PhD
Objective: Evaluate and establish the relationship of five critical X-ray time points during multilevel posterior column osteotomy (MPCO) spinal deformity realignment in patients with adult spinal deformity and measure pre- to post-operative outcome longitudinally.
Eligibility Criteria: Adults who are a candidate for posterior-only surgery for spinal deformity
ClinicalTrials.gov Identifier: NCT05154825
Study Status: Enrolling
Contact: Peter Chase, MS, pchase@health.ucsd.edu, (858) 657-7651
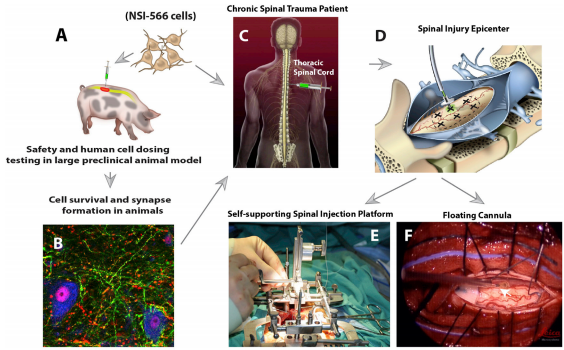
Safety Study of Human Spinal Cord-derived Neural Stem Cell Transplantation for the Treatment of Chronic Spinal Cord Injury
Principal Investigator: Joseph Ciacci, MD
Objective: Evaluate the safety of human spinal cord-derived neural stem cell (HSSC) transplantation for the treatment of chronic spinal cord injury
Eligibility Criteria: Adults with chronic spinal cord injury
ClinicalTrials.gov Identifier: NCT01772810
Study Status: Enrolling
Contact: alphastemcellclinic@ucsd.edu, (844) 317-STEM (7836)
Cerebrovascular
Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial – Hemodynamics (CREST-H)
Principal Investigator: Alexander Khalessi, MD, MBA
Objective: Determine whether cognitive impairment attributable to cerebral hemodynamic impairment in patients with high-grade asymptomatic carotid artery stenosis is reversible with restoration of flow.
Eligibility Criteria: Patients who are enrolled in the CREST-2 study
ClinicalTrials.gov Identifier: NCT03121209
Study Status: Enrolling
Contact: Peter Chase, MS, pchase@health.ucsd.edu, (858) 657-7651
Embolization of the Middle Meningeal Artery with ONYX™ Liquid Embolic System In the Treatment of Subacute and Chronic Subdural Hematoma (EMBOLISE)
Principal Investigator: J. Scott Pannell, MD
Objective: Evaluate the safety and efficacy of embolization of the middle meningeal artery (MMA), a minimally invasive procedure, for the treatment of symptomatic subacute or chronic subdural hematoma.
Eligibility Criteria: Adults diagnosed with chronic or subacute hematoma
ClinicalTrials.gov Identifier: NCT04402632
Study Status: Enrolling
Contact: Peter Chase, MS, pchase@health.ucsd.edu, (858) 657-7651
Lumbar Drain vs Extraventricular Drain to Prevent Vasospasm in Subarachnoid Hemorrhage
Principal Investigator: Alexander Khalessi, MD, MBA
Objective: Evaluate whether extraventricular drain (EVD) or lumbar drain (LD) prevents clinical vasospasm, decreases subarachnoid blood, shortens overall ICU stay or reduces the need for a permanent ventriculoperitoneal shunt. The conclusions of this study may identify an optimal treatment modality to benefit all future patients with ruptured intracranial aneurysms.
Eligibility Criteria: Adults with grade II, III, IV subarachnoid hemorrhage or patients with aneurysmal SAH with radiographic evidence
ClinicalTrials.gov Identifier: NCT03065231
Study Status: Enrolling
Contact: David Santiago-Dieppa, MD, drsantiagodieppa@ucsd.edu, (818) 357-1766
Assessing Neurocognition After Cerebrovascular Intervention
Principal Investigator: Alexander Khalessi, MD, MBA
Objective: Provide prospective evidence to identify the extent to which carotid stenosis and hypoperfusion of the brain results in diminished neurocognitive performance, and see if serum biomarkers before and after stenting correlate with these findings
Eligibility Criteria: Adults with ultrasound evidence of carotid stenosis; in which the patient has either 50% or greater symptomatic carotid stenosis or 70% or greater asymptomatic carotid stenosis
ClinicalTrials.gov Identifier: NCT03344276
Study Status: Enrolling
Contact: Arvin Wali, MD, awali@ucsd.edu, (714) 928-2722
